
Double replacement reactions swap cations or the anions, but not both. Subscribe for more great chemistry videos! Double replacement Reaction (Double displacement Reaction)Ī double replacement reaction, aka double displacement reaction, exchanges ionic species in two compounds to form two completely new compounds, with the exchange of ions between the reactants. Video showing the single displacement of hydrogen by elemental samarium. The decomposition of hydrogen peroxide results in water and oxygen gas. A common example of a decomposition reaction is the decomposition of hydrogen peroxide. Reactions that require an input of energy are endothermic. Unlike synthesis reactions, decomposition reactions require energy to break the bonds present in the reactant. Here is the general equation that represents this type of reaction: Sodium and chlorine ions interact to form sodium chloride.Ī decomposition reaction occurs when the reactant breaks down into simpler products. A typical example of a synthesis reaction is the formation of table salt. Reactions that release energy are considered exothermic. In most cases, synthesis reactions release energy. The general equation represents this type of reaction: The product created is different from both of the reactants. Types of Chemical Reactions Synthesis ReactionĪ synthesis reaction occurs when two reactants interact to form one product. The Four Basic Types of Chemical Reactions
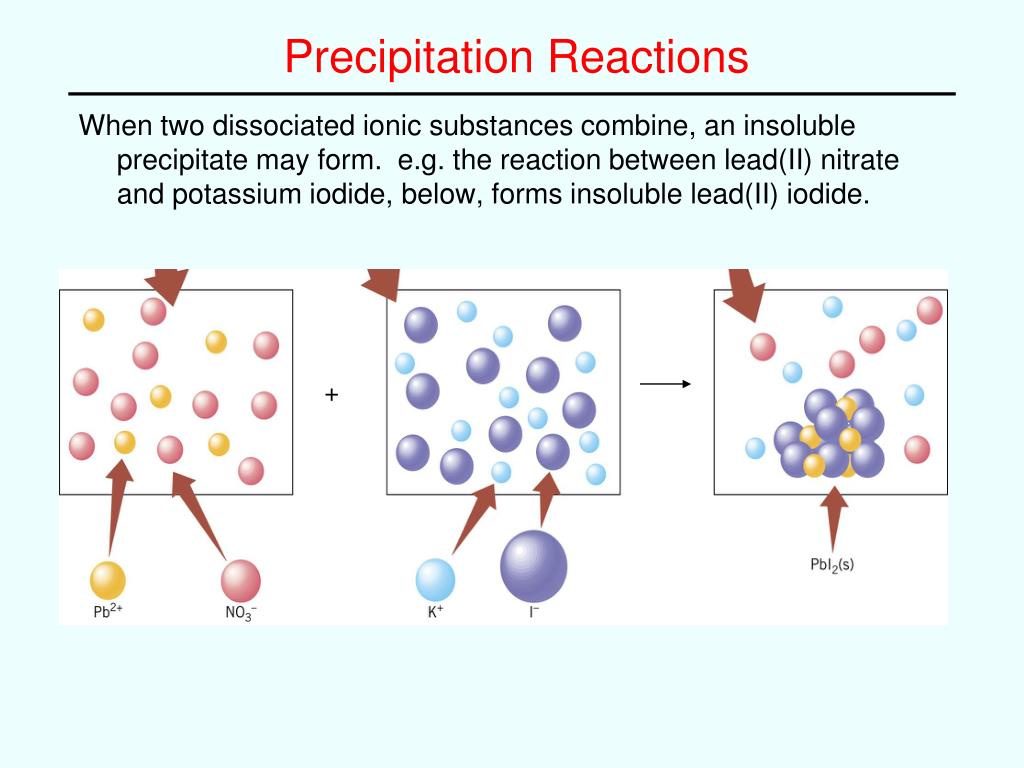
neutralization (acid base reaction)- a double replacement reaction in which an acid reacts with a base to form water and salt.double replacement reaction or double displacement reaction – a reaction in which the cationic or the anionic species switch places, creating two new products.single replacement reaction or single displacement reaction– a reaction that occurs when a new compound is formed when one element is substituted for another element in a compound, creating a new element and a new compound as products.combustion reaction – when a substance reacts with oxygen, forming light and heat in the form of fire.decomposition reaction– a reaction that occurs when a compound breaks down into two or more atoms.

synthesis reaction- a reaction that occurs when two atoms interact to form one atom.
We also discuss what is a combustion reaction, precipitation reaction, and acid base reaction. This article will cover the main classifications of chemical reactions: synthesis reaction, decomposition reaction, single replacement reaction (single displacement reaction), and double replacement reaction (double displacement reaction). Types of Chemical Reactions : Core Concepts


 0 kommentar(er)
0 kommentar(er)
